Silver Nitrate Standard Solutions – How to make them, and SAVE MONEY!
How to Make your own Silver Nitrate Standard Solutions
| Normal | (Molar) | High Purity | Distilled or D.I. |
| Solution | Desired | Silver Nitrate | Water to Make |
| 0.0141 | N | 2.395 grams | 1 Liter |
| 0.0171 | N | 2.904 grams | 1 Liter |
| 0.0192 | N | 3.261 grams | 1 Liter |
| 0.020 | N | 3.397 grams | 1 Liter |
| 0.025 | N | 4.246 grams | 1 Liter |
| 0.0282 | N | 4.790 grams | 1 Liter |
| 0.045 | N | 7.644 grams | 1 Liter |
| 0.050 | N | 8.493 grams | 1 Liter |
| 0.1 | N | 16.987 grams | 1 Liter |
| 0.141 | N | 23.951 grams | 1 Liter |
| 0.153 | N | 25.990 grams | 1 Liter |
| 0.171 | N | 29.047 grams | 1 Liter |
| 0.2 | N | 33.974 grams | 1 Liter |
| 0.25 | N | 42.467 grams | 1 Liter |
| 0.282 | N | 47.903 grams | 1 Liter |
| 0.5 | N | 84.935 grams | 1 Liter |
| 1.0 | N | 169.870 grams | 1 Liter |
| 2.0 | N | 339.740 grams | 1 Liter |
| 3.0 | N | 509.610 grams | 1 Liter |
| 4.0 | N | 679.480 grams | 1 Liter |
| Volumetric Flasks | Meniscus |
 |
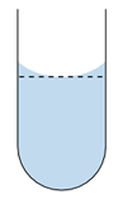 |
| Volumetric Flasks look like this. There are many sizes and styles. 1000 ml equals 1 Liter |
Add Water until you have reached the mark on the flask like the example above. |
You can save a lot of money by making your own Silver Nitrate Standard Solutions! It’s Easy!
What you need
1 – A Volumetric Flask. ( from a chem store, catalog, or eBay )
2 – An Accurate Scale. ( there are very good ones on eBay )
3 – De-Ionized -or- Distilled Water. ( don’t use tap water! )
4 – High Purity Silver Nitrate. ( you can buy it on this website )
Instructions
1 – Find the concentration of the solution you want to make, from the table, above.
2 – Carefully weigh the exact amount of High Purity Silver Nitrate you need.
3 – Add the Silver Nitrate powder to the empty Volumetric flask.
4 – Add about half of the water, swirl the flask to dissolve all of the Silver Nitrate.
5 – Carefully add the rest of the water, until the water Meniscus reaches the mark on the flask.
6 – That’s it. You can pour your solution into another bottle at this point.
Important: Use High Purity Silver Nitrate only. ( we sell High Purity Silver Nitrate )
Important: Use De-Ionized -or- Distilled Water only. ( never use tap water )
Important: Store your solution capped. Don’t let it evaporate – that will concentrate it!
HINT: Store your standard solutions in Glass Containers. (not soft plastic)
HINT: Silver Nitrate solutions can be stored in type PETE 1 (polyethylene terephthalate ester) Plastic.
HINT: The solutions are not light sensitive. You don’t need dark bottles.
HINT: After pouring your solution from the Volumetric flask, add a pinch of salt to the flask, then rinse and drain at least 3 times.
HINT: Rinse your Volumetric Flask with Distilled Water, then let it dry, before storing it.
HINT: If you don’t use High Purity Silver Nitrate, your solution won’t be colorless, like it should be.
HINT: With Silver Nitrate Standard Solutions, Normal and Molar have the same meaning. (because it’s Valence is +1)
HINT: 100 gram Capacity Digital Scales, accurate to 0.01 grams, can be found on eBay for under $20!
KNOW THIS: One Mole of Silver Nitrate weighs 169.87 grams. So a 1.0N solution is: 169.87 grams diluted to a volume of 1 Liter.
KNOW THIS: One Mole of Silver Nitrate weighs 169.87 grams. So a 0.1N solution is: 16.987 grams diluted to a volume of 1 Liter.
